Project Summary
Quality CDMO’s website was created to present their comprehensive pharmaceutical contract services — including product development, formulation, manufacturing, packaging, testing, and quality assurance — to potential clients within the pharmaceutical and personal care industries.
The objective was to clearly articulate the company’s capabilities for drug formulation, scalable cGMP-compliant manufacturing, and regulatory support — helping businesses understand how Quality CDMO can bring complex products from concept to production.
Key highlights:
- Full-spectrum CDMO services: development, manufacturing, packaging, and testing
- Capability to produce liquid, semi-solid, and finished consumer products
- Focus on quality assurance and regulatory compliance with current good manufacturing practices
- Facility registered with the FDA and Texas Department of Health
-
Effectively communicating the broad range of technical services (from early formulation to finished product manufacturing).
-
Presenting detailed pharmaceutical manufacturing capabilities without overwhelming the audience.
-
Reinforcing quality and regulatory compliance credentials in a heavily regulated industry.
-
Balancing technical content with simple lead capture pathways for prospects.
-
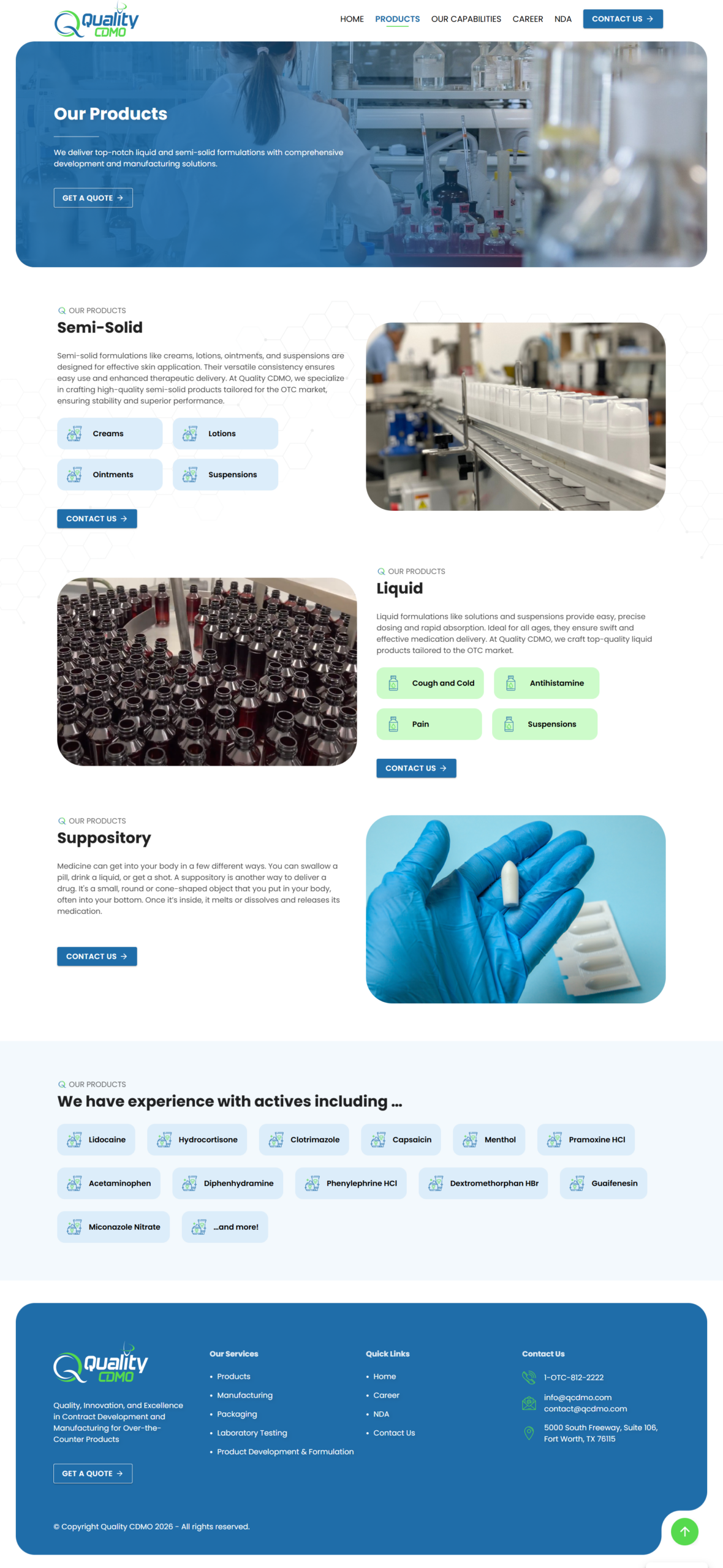
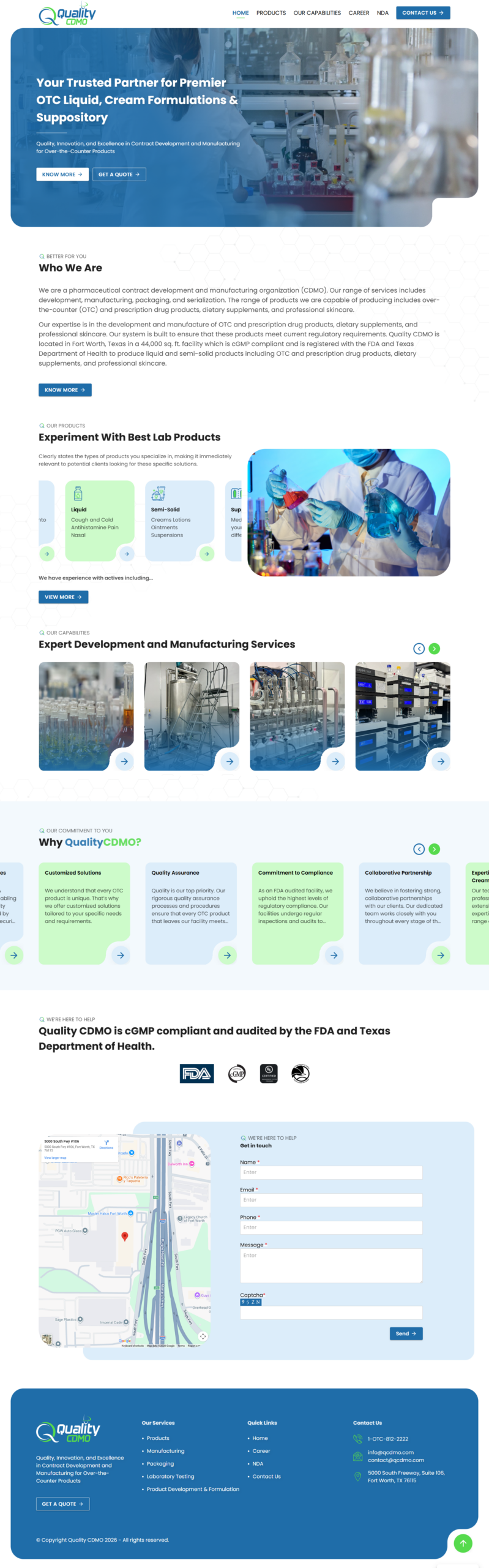
Clear Capability Breakdown
Organised content into core services — formulation & product development, manufacturing operations, laboratory testing, and packaging — to guide users through the lifecycle of pharmaceutical product creation.
-
Service-First Navigation
Ensured site navigation allowed potential clients to quickly locate specific capabilities — such as semi-solid product manufacturing and serialization services — without needing deep technical expertise upfront.
-
Regulatory Emphasis
Highlighted the facility’s cGMP compliance and FDA registration to build credibility with pharmaceutical and healthcare clients.
-
Lead Generation Focus
Prominent “Request a Quote” and “Contact Us” CTAs throughout the site structure to convert interest into business inquiries.
- Wordpress
Make your vision into Reality with Our Expert Team
Our experts learn and research to understand your project idea, discuss it, provide an analysis, insight, and estimates and duration to complete the project.